There are no items in your cart
Add More
Add More
| Item Details | Price | ||
|---|---|---|---|

1. Stock name: Jubilant Ingrevia Ltd.
Pattern: Double bottom pattern
Time frame: Daily
Observation:
Listed on March 19, 2021, the stock initially surged until October 2021, hitting its peak during that phase. Following this, there was a downturn until February 2022, after which it entered a sideways trend. Notably, a double bottom pattern was formed on the daily chart during this phase. On August 23, 2023, the stock broke out from this pattern with substantial trading volumes, propelling it upwards. As per technical analysis, if the stock is able to sustain this momentum it may lead to further upward movement.

You may add this to your watch list to understand further price action.
Disclaimer: This analysis is purely for educational purpose and does not contain any recommendation. Please consult your financial advisor before taking any financial decision.
--------------------------------------------------
2. Stock name: Jindal Worldwide Ltd.
Pattern: Double bottom pattern and retest
Time frame: Daily
Observation:
Between September 2021 and January 2023, the stock displayed an ascending trajectory which was followed by a descent. Subsequently, it has formed a bullish double bottom pattern. Notably, a breakout from the pattern has emerged on August 11, 2023, accompanied by substantial trading volumes. The stock has moved a little upwards after the breakout. Currently, the stock is in the process of retesting the breakout. As per technical analysis, if the stock is successful in making a bounce back from this retest, it may move furhter upwards.
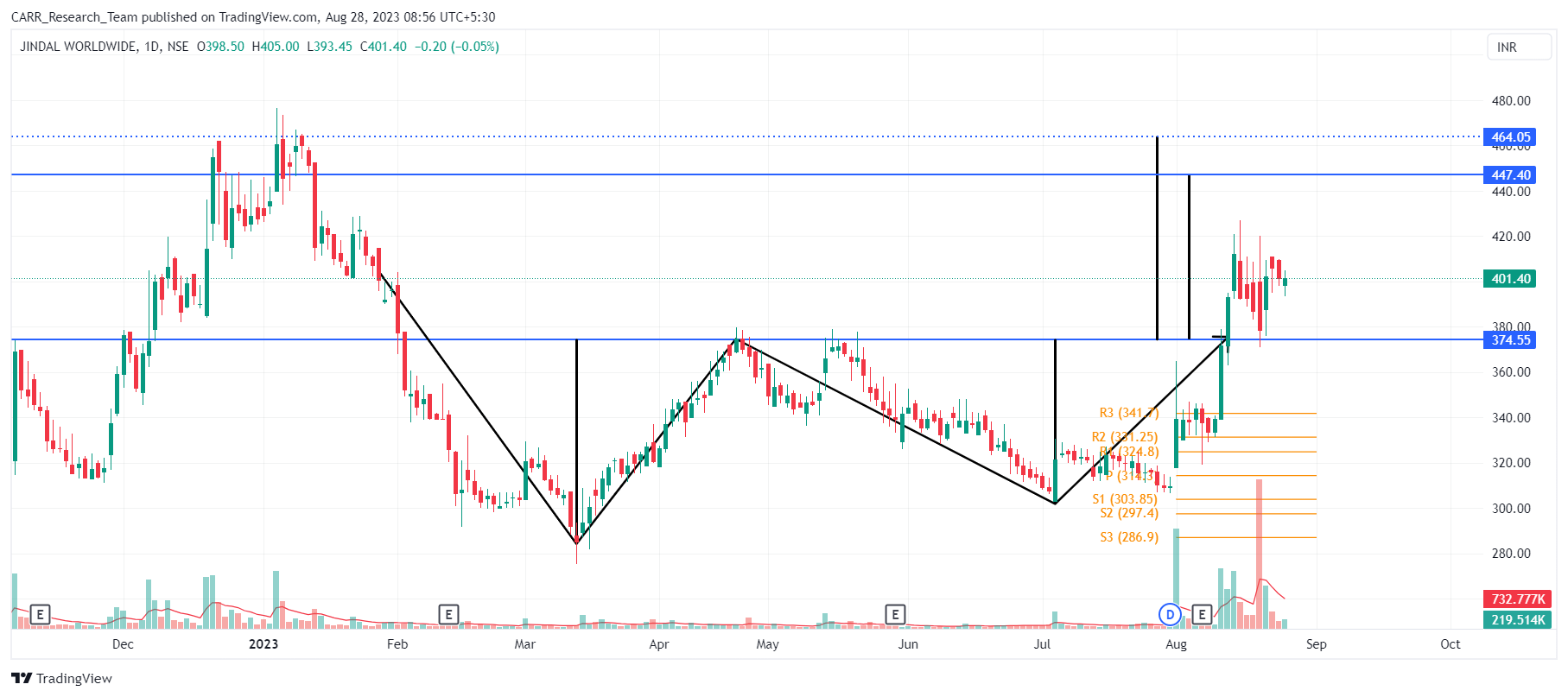
You may add this to your watch list to understand further price action.
Disclaimer: This analysis is purely for educational purpose and does not contain any recommendation. Please consult your financial advisor before taking any financial decision.
News for the day:
1) The Supreme Court has granted permission for a forensic audit of Ruchi Soya Industries, now under Patanjali Ayurved, following a request by IDBI Bank, the lead lender in a consortium, with the former management required to cooperate in the process.
2) Vedanta has emerged victorious in an arbitration against the Indian government's claim for a greater payout related to Rajasthan oil and gas fields, with the tribunal upholding Vedanta's position that the additional payment wasn't required under the Production Sharing Contract terms. Vedanta is assessing the award's financial implications.
3) Lupin has obtained US FDA approval to distribute a generic medication for lung disease treatment in the US market. The approval encompasses Pirfenidone tablets in 267 mg and 801 mg strengths.